Elisa Kit for Follicle Stimulating Hormone (FSH)
Overview Product Description EVANCARE Diagnostic FSH Rapid Test Kits For professional and in vitro diagnostic use only.
Send your inquiryDESCRIPTION
Basic Info
| Model NO. | EC-FSH |
| Function | Early Detection |
| Display | Screening |
| Format | Strip+Bottle |
| Name | Fsh Test Kit |
| Equipment | by Eye or Machine |
| Reaction Time | 5-10 Seconds |
| Accuracy | 99.7% |
| Delivery Time | 3-5 Days |
| Temperature | 4-30 Degree |
| Storage | Room Temperature |
| Transport Package | Carton |
| Specification | 64*50*44 cm |
| Trademark | evancare or OEM |
| Origin | China |
| HS Code | 3002150090 |
| Production Capacity | 100000PCS/Day |
Product Description
Product Description
EVANCARE Diagnostic FSH Rapid Test KitsFor professional and in vitro diagnostic use only.
[INTENDED USE]The FSH Menopause Rapid Test Strip/cassette/Midstream is a rapidchromatographic immunoassay for the qualitative detection of Follicle Stimulating Hormone (FSH) in urine to aid in the detection of menopause.
[PRINCIPLE] Follicle Stimulating Hormone(FSH) is excreted by the pituitary gland. During the follicular phase of the menstrual cycle, FSH fosters the growth and maturation of a woman's eggs. If a woman experiences infertility or approaches menopause, it may be a sign of a low ovarian egg supply - and this is indicated by a high level of FSH.With infertility issues, the FSH is released in higher-than-normal amounts to stimulate the ovaries into producing a mature egg and more estrogen. The FSHMenopause Rapid Test Cassette is a test kit for the determination of human Follicular Stimulating Hormone concentration in urine specimens.
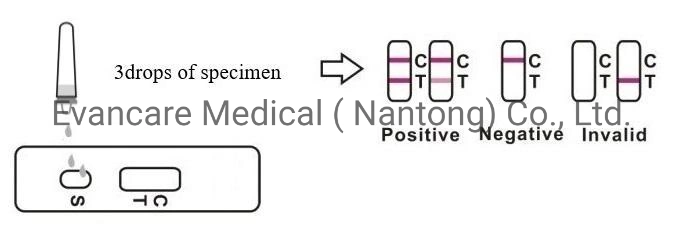
The test reagent is exposed to urine, allowing urine to migrate through the absorbent test cassette. The labeled antibody-dye conjugate binds to the FSH in the specimen forming an antibody-antigen complex. This complex binds to the anti-FSH antibody in the test region (T) and produces a color line. In the absence of FSH, there is no color line in the test region (T). The reaction mixture continues flowing through the absorbent device past the test region (T) and control region (C).Unbound conjugate binds to the reagents in the control region (C), producing a color line, demonstrating that the test cassette is functioning correctly. The test cassette can accurately detect your FSH surge when the concentration of FSH is equal to or greater than 25mIU/ml. .
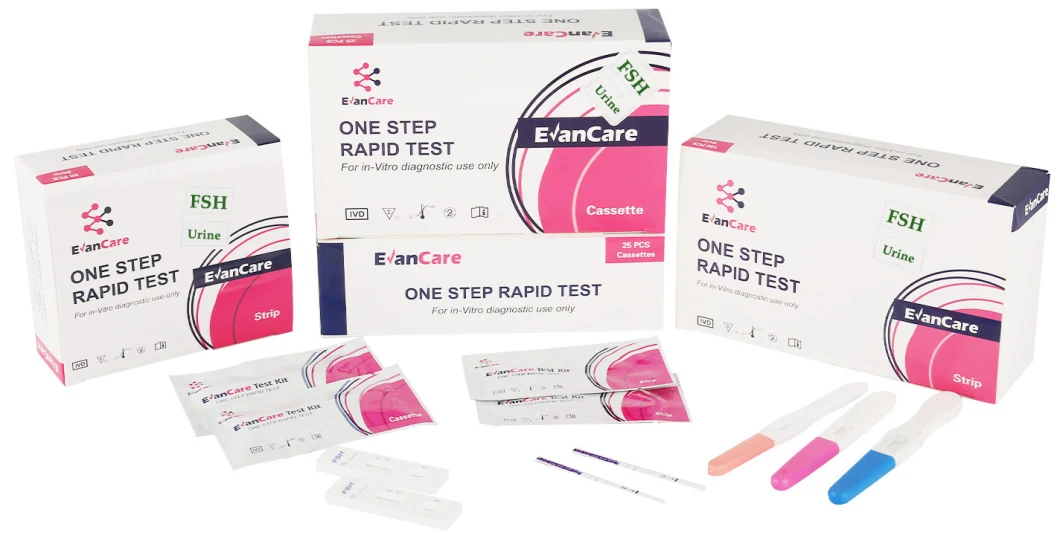
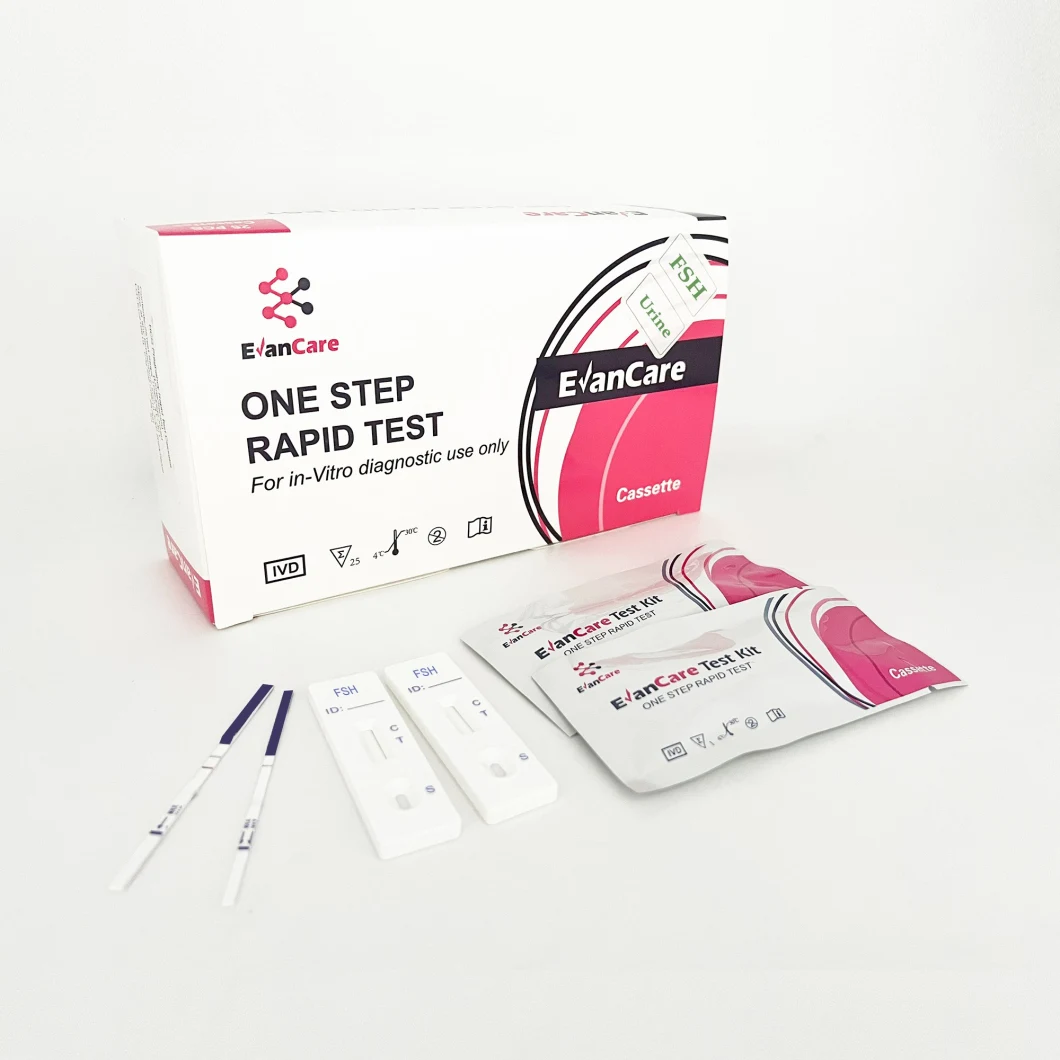
Detailed Photos
Positive: *Two lines appear. One colored line should be in the control region (C), and another apparent colored line adjacent should be in the test region (T). Negative: One colored line appears in the control region (C). No line appears in the test region (T). Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test using a new test cassette. If the problem persists, discontinue using the lot immediately and contact your local distributor.Item No. | EVANCARE Diagnostic FSH Rapid Test Kits |
Contents | Each bag:1 test cassette and 1 desiccant(cassette) 25 cassettes+1 Buffer /box antigen test We can put instruction as your requirement |
Format / Size | Cassette:2.5mm 3.0mm 4.0mm |
Methodology | Sandwich Colloidal Gold |
Assay Type | Qualitative Detection |
Specimen | Antigen (Urine), |
Indicator | FSH Test Cassette |
Storage Temperature | 4-30 degree centigrade |
Expiry date | 3 years |
Certificate | CE,ISO,FREE SALE,FDA... |
Detection Limits | Home or Professional Use |
Accuracy | > 99.99% |
| Reaction Time | 3-5 min |
| Delivery | 5-7 days |
| OEM Service | Available |
| Shipping Method | by air or sea or international express |
Company Profile
Certifications
Product Procedure
Activity Exhibition