CE-Marked Neutralizing Antibody Rapid Test Kit (ELISA) for 2019 New Infectious Virus
Neutralizing Antibody Detection Kit(ELISA) Product Description Neutralizing Antibody Detection Kit(ELISA ) is an indirec
Send your inquiryDESCRIPTION
Basic Info
| HS Code | 1108966068 |
| Production Capacity | 10, 000 Boxes / Day |
Product Description
Neutralizing Antibody Detection Kit(ELISA)Product Description
Neutralizing Antibody Detection Kit(ELISA ) is an indirectenzyme-linked immunoassay (ELISA) for quantitative determination of virus Ncutralizing Antibody in plasma or serum. This kit is used for assisted diagnosis.
Product Features
1. High specificity
2. High sensitivity
3. High stability
Priciple of the assay
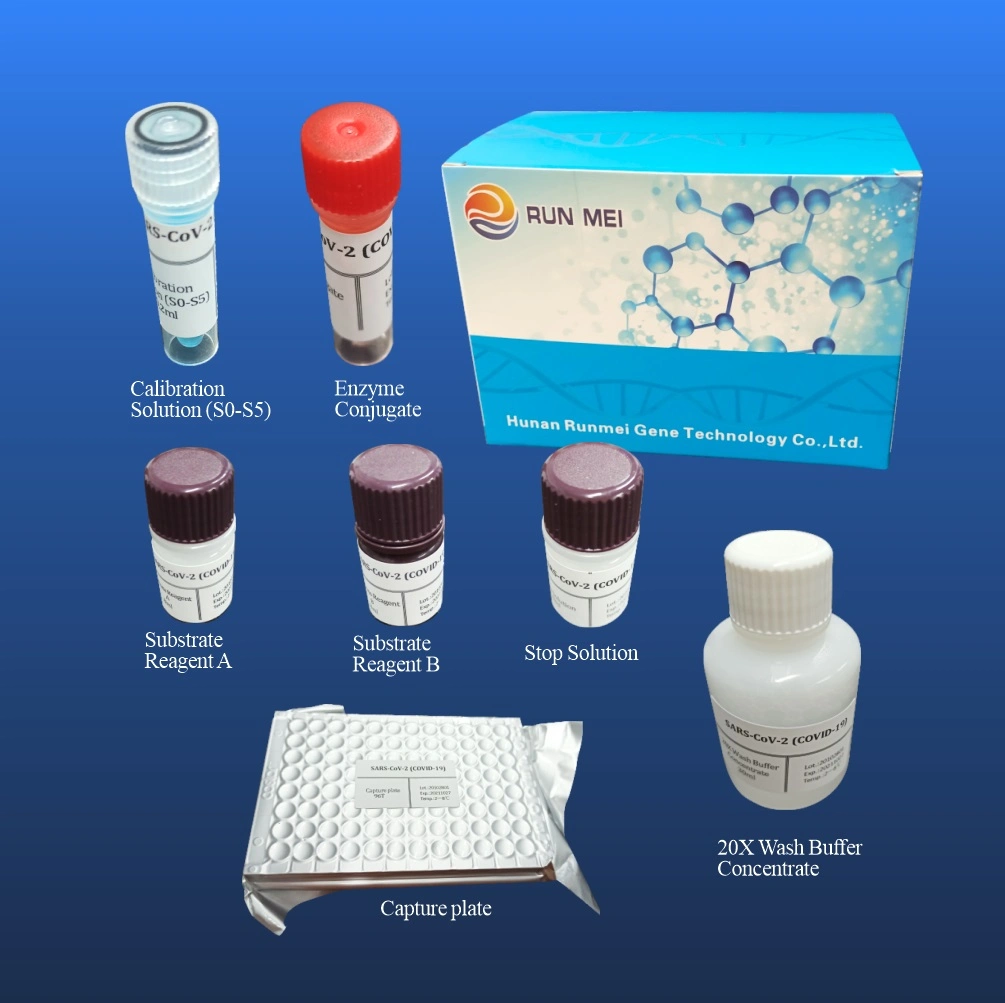
Virus Neutralizing Antibody Detection Kit(ELISA ) is for rapid Nab(neutralizing antibody) screening, which can mimic the virus neutralization process. The kitcontains two key components: the Horseradish peroxidase(HRP) conjugated recombinant virus RBD fragment (HRP-RBD) and the human ACE2 receptor protein (hACE2). The protein-protein interaction between HRP-RBD and hACE2 can be blocked by neutralizing antibodiesagainst virus RBD.
First, the samples and controls are pre-incubated with the HRP-RBD to allow the binding of thecirculating neutralization antibodies to HRP-RBD. The mixture is then added to the capture platewhich is pre-coated with the hACE2 protein.The unbound HRP-RBD as well as any HRP-RBDbound to non-neutralizing antibody will be captured on the plate,while the circulatingncutralization antibodies_HRP-RBD complexes remain in the supernatant and get removedduring washing.After washing steps,TMB solution is added.By adding Stop Solution,thereaction is quenched. This final solution can be read at 450 nm in a microtiter plate reader. Theabsorbance of the sample is inversely dependent on the titer of the anti-virus neutralizingantibodies.
Sample requirements
Applicable sample types: Serum or plasma.
Handling and storage of samples:
·Follow procedures within your laboratory to avoid cross contamination of patient specimens.·For human/animal plasma, treat blood with anticoagulant such as citrate,EDTA or heparin.- Sample inactivate under 56°C for 30min can be used.
· Samples may be stored at room temperature (15-30°C) for no longer than 8 hours. If the test willnot be completed within 8 hours, refrigerate the serum samples at 2-8°C for no longer than 8 days.·Run assay immediately, otherwise store samples below -18°C.Avoid repeated freeze-thaw cycles.
·As an alternative to the above, sample stability may be established by each laboratory.
| Name | Number | Packing unit |
| Neutralizing Antibody Detection Kit(ELISA ) | 96 testing | kit |
| Unit | Piece | Volume(CM) | Weight(KG) |
| Per box | 50 | 15.6*11*11 | 0.35 |
| per carton | 48*50 | 65*40*45 | 16.66 |
| per pallet | 14*48*20 | 120*105*135 | 233.24 |

Company Profile
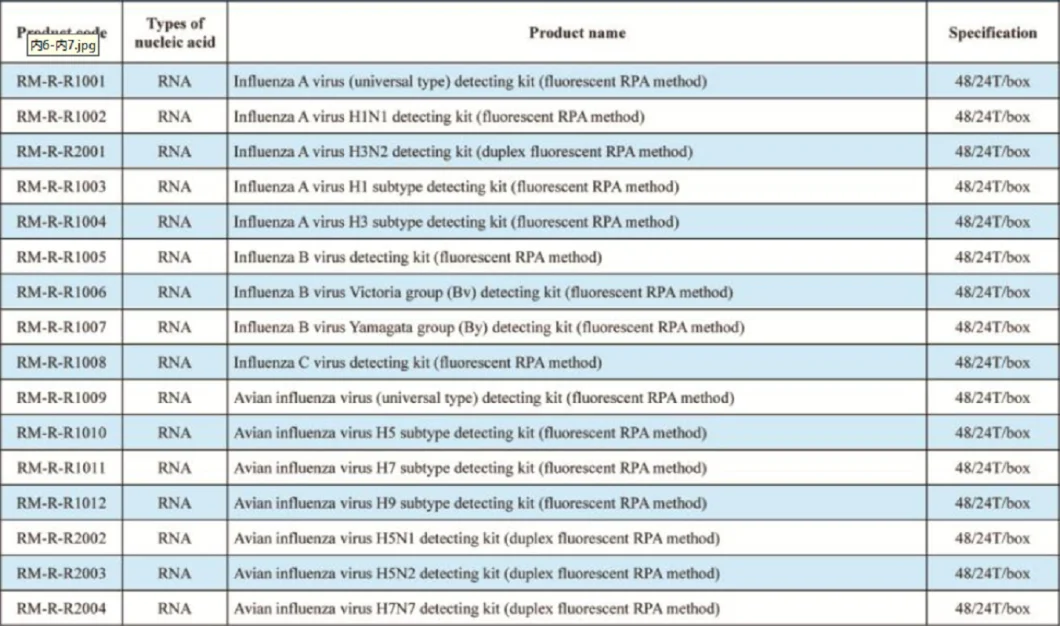
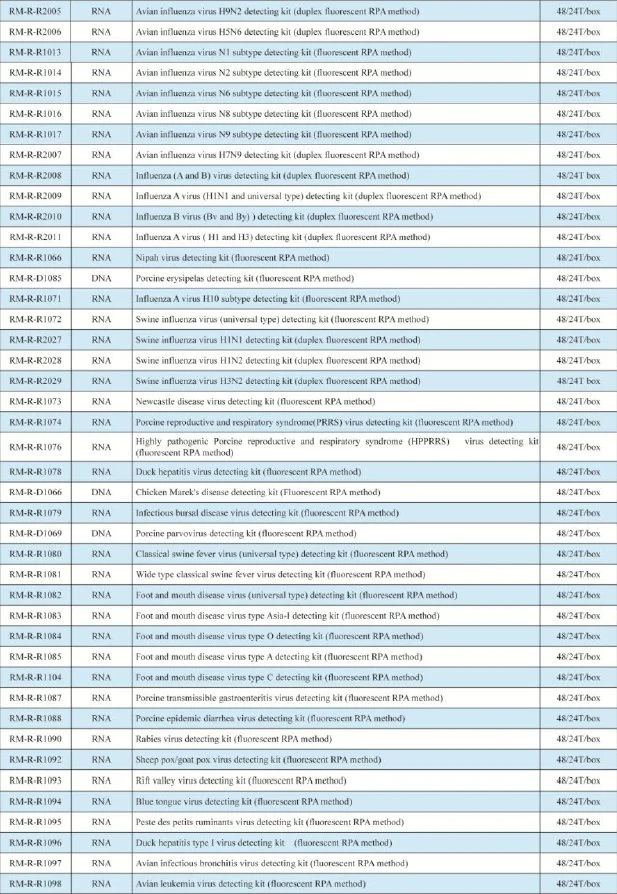
Hunan Runmei Gene Technology is a high-tech enterprise dedicated to thedevelopment of the gene detection products and the construction of big data serviceplatforms led by a team of doctors at home and abroad. Our company's strategic goal isto base on China and radiate the world, and to solve the pain points and difficulties of theindustry and create value for human beings as our corporate purpose. At present, ourcompany has completed the construction of product systems for pathogen biology fluoresencequantitative PCR detection kits, pathogen biology ELISA detection kits and pathogen biologyimmune colloidal gold detection kits.

Certification